XAS Applications in Biology
How does Ag (silver) kill bacteria? – It "hooks“ up to cysteine, enzymes or DNA and disrupts the metabolism and the reproductive cycle.
Silver is quite an aggressive metal in the metabolic system. However, Ag can only occupy "biological" sites with amino acids which are the components of proteins or with the nucleotides which are components of DNA, RNA (Fig. 1). Data from Ag impregnated bacterial cells at Ag L3 edge confirms this. Linear combination fitting resulted in perfect fits using just these 4 Ag-amino acid compounds (Fig. 2).
G. L. Bovenkamp, U. Zanzen, K. Sai Krishna, J. Hormes, A. Prange (2013) X-Ray Absorption Near-Edge Structure (XANES) Spectroscopy Study of the Interaction of Silver Ions with Staphylococcus aureus, Listeria monocytogenes, and Escherichia coli. Appl. Environ. Microbiol. 79:20 6385-6390; DOI:10.1128/AEM.01688-13
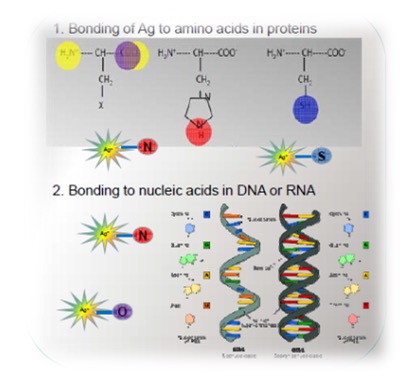
Figure 1: Possible Ag bonding sites in cell
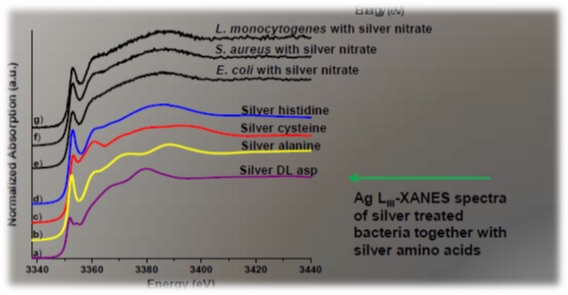
Figure 2: Results: Ag L3 XANES spectra of bacterial cells with Ag and Ag-amino acid reference compounds
Nanoparticles used for cancer treatment
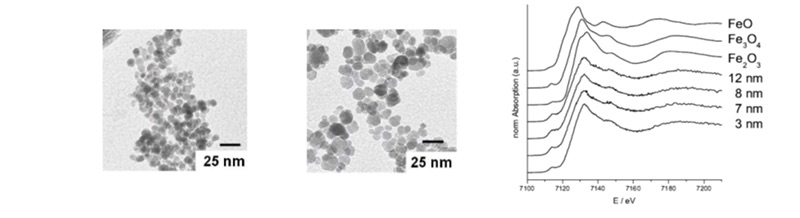
The optimization of iron oxide nanoparticles for the enhancement of contrast of tumor cells for MRT was investigated . Can the contrast be enhanced by changing the magnetic moment of the ion-oxide nanoparticles? The biofunctionalization was achieved using LHRH (Luteinizing Hormone Releasing Hormone) ligands. Smaller nanoparticles are taken up much better by the cells but the magnetic moment (and therefore the contrast) is better for the bigger nanoparticles. Figure 3a and 3b show the TEM of the nanoparticles. In Figure 3c the XANES spectra show the high similarity of the nanoparticles inside the cells with magnetite and confirm that they did not change during uptake.